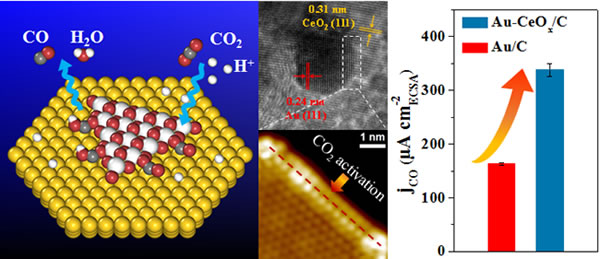
Recently, researchers from the State Key Laboratory of Catalysis of Dalian Institute of Chemical Physics, Chinese Academy of Sciences Wang Guoxiong, Yang Fan and Academician Bao Yanhe of the Chinese Academy of Sciences have made new progress in the study of enhanced electrocatalytic reduction of carbon dioxide at the metal-oxide interface. The relevant results were published a few days ago. Published on the American Chemical Society (J. Am. Chem. Soc. 2017, 139, 5652). Electrocatalytic reduction of carbon dioxide, using clean electric energy such as renewable electric power or surplus nuclear power as the energy source, converts carbon dioxide into carbon monoxide, formic acid, hydrocarbons, alcohols and other high value-added fuels and chemicals in a single reaction under mild reaction conditions. Efficient conversion of carbon dioxide and effective storage of clean electricity. Currently, designing high-efficiency catalysts to reduce the overpotential and increase the selectivity of the reaction is a very challenging hot topic in the study of carbon dioxide electrocatalytic reduction. The research team is based on the preliminary study of carbon dioxide electrocatalytic reduction (J. Am. Chem. Soc. 2015, 137, 4288; Nano Energy 2016, 27, 35; Chem. Sci. 2017, 8, 2569; Nano Res. 2017, Doi: 10.1007/s12274-017-1514-6) based on a full understanding of the metal-oxide interfacial catalytic confinement effect (Science 2010, 328, 1141; Nat. Sci. Rev. 2015, 2, 183; Nat. Commun 2017, 8, 14459) Designed and synthesized carbon-supported Au-CeOx catalysts with metal-oxide interfacial structure. The Au-CeOx interface and carbon dioxide were studied under the condition that the Au nanoparticle size and morphology were the same in different catalysts. The intrinsic correlation of electrocatalytic reduction performance. At -0.89 V (vs. RHE), the farnesic efficiency of carbon monoxide on the Au-CeOx catalyst reaches 89.1%, much higher than that of Au alone (59.0%) or CeOx catalyst (9.8%), and the current density of carbon monoxide produced is Au. 1.6 times. By constructing a CeOx/Au(111) model catalyst and conducting in-situ investigations using high-resolution scanning tunneling microscopy and synchrotron radiation energy spectra, it was found that the Au-CeOx interface significantly promotes the adsorption and activation of carbon dioxide at the interface of CeOx, and the presence of water helps. The reduction of CeOx surface is stable with the adsorption of carbon dioxide species on the surface. Density functional calculations further indicate that the Au-CeOx interface helps stabilize the key intermediate species, *COOH, in the subsequent hydrogenation process, thereby promoting carbon monoxide generation and desorption. This interface-enhanced electrocatalytic reduction of carbon dioxide has been further confirmed in the Ag-CeOx catalytic system, indicating the universality of the metal-oxide interface catalytic system in the electrocatalytic reduction of carbon dioxide. The research results provide a new way to regulate the electrocatalytic reduction of carbon dioxide, enriching and expanding the nano-limitation catalytic concept proposed by the research team. The above research work has been funded by the National Natural Science Foundation of China, the National Key Research and Development Program, and the pilot projects of the Chinese Academy of Sciences. Smart Lighting,Smart Led Lights,Colorful Smart Light,Led Piano Floor Tile Light Shenzhen Huangtai Photoelectric Co.,Ltd. , https://www.huangtailightstrip.com