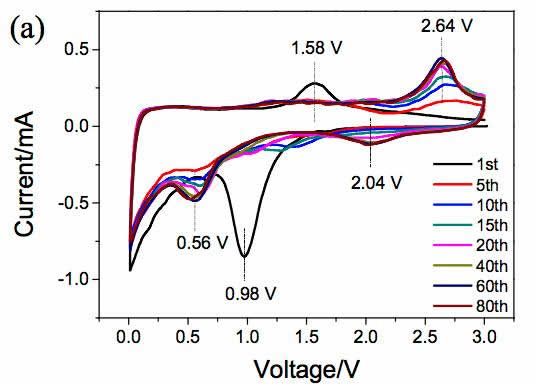
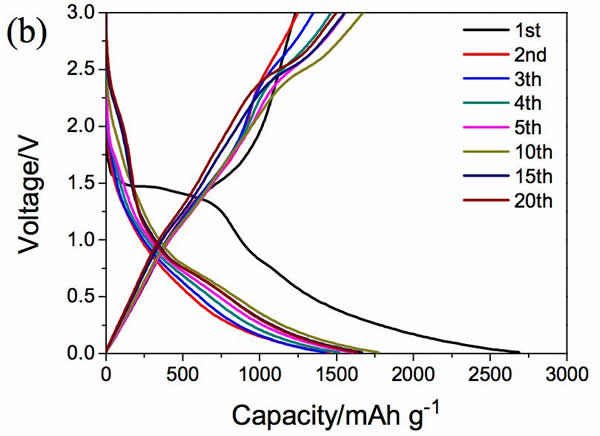
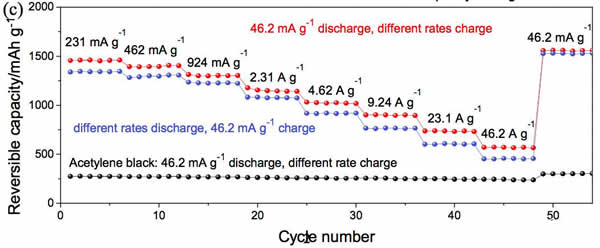
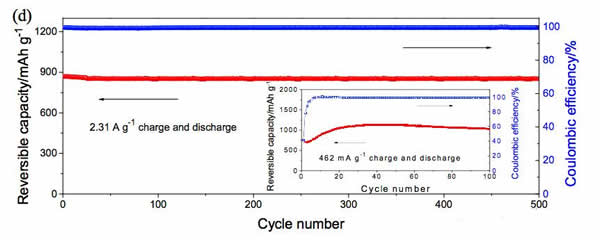
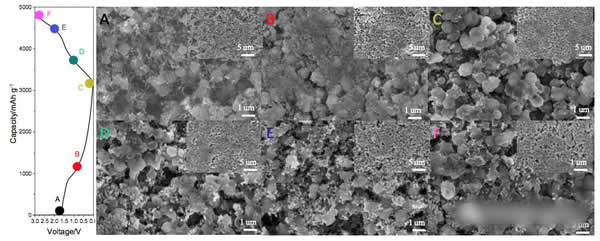
Lithium ion batteries are currently the most widely used chemical energy storage power sources. In general, lithium ion batteries are mainly composed of a transition metal oxide positive electrode and a lithium-insertable negative electrode such as graphite, and the positive and negative electrodes are separated by a porous polymer separator. Between the organic electrolyte filled, this constitutes the basic structure of lithium-ion batteries. Among them, positive and negative electrode materials can be used to supply energy to the battery. At present, commercial positive and negative electrode materials for lithium ion batteries are all made of inorganic active materials such as NCA, NCM of positive electrode, graphite, hard carbon of negative electrode, etc. With the continuous development of high-specific energy batteries, these traditional inorganic materials are gradually lacking in capability. We urgently need us to look for a new generation of electrode materials. However, the types of inorganic compounds are limited. Therefore, our choice is not many. In contrast, the types of organic compounds are very rich. Only three elements, carbon, hydrogen, and oxygen, can form millions of compounds. This is one of the few that has electrochemical activity, providing us with many options. Recently, Honghe Zheng and Yan Wang of Soochow University proposed a high capacity (1500mAh/g at 46.2mA/g current density) and high power (570.8mAh/capacity at 46.2A/g current density). g) and long-life (at a current density of 2.31A/g, the cycle capacity of 500 cycles up to 570.8mAh / g) characteristics of organic lithium ion battery anode material - maleic acid. Yan Wang believes that the reason why maleic acid has such excellent performance is mainly due to its very small volume expansion and unique lithium insertion mechanism, so it is very suitable for applications with high power requirements. Yan Wang used maleic acid in the experiment. The following picture shows the cyclic voltammogram of maleic acid material. The scanning speed is 0.1mV/s. From the curve we can see that near the 0.98V during the first reduction process. A very wide reduction peak appears, but this current peak does not reappear during the subsequent cycle. There are two reasons for this phenomenon: 1) Maleic acid reacts with solvated Li to produce lithium maleate; 2) Another reason is the formation of SEI film on the negative electrode surface. During the oxidation, an oxidation current peak appeared at 1.58V, but this current peak disappeared during the subsequent cycle. In the subsequent cycle, there were a total of three reduction peaks, 2.04, 0.56, and 0V, respectively, and an oxidation peak at 2.64 V. After 20 cycles, the cycle curve stabilized. The charge and discharge curves of maleic acid during the first 20 cycles are shown in the following figure. It can be seen that the discharge capacity is 2688.9 mAh/g, the charge capacity is 1233.5 mAh/g, and the first efficiency is 45.9% during the first charge/discharge. However, the Coulomb efficiency increased to 86% during the second cycle, and the capacity of the subsequent cycle gradually increased and stabilized at 1500mAh/g, which is not only higher than other organic anode materials, but also higher than that of graphite. The capacity of 372mAh/g is significantly increased. The following figure shows the rate performance of maleic acid materials. From the figure, it can be seen that at a current density of 4.62 A/g, the capacity of the material can reach 1025 mAh/g, at a current density of 23.1 A/g and 46.2 A/g. The material capacity can reach 736.7mAh/g and 570mAh/g, respectively, which are much higher than graphite materials, indicating that the maleic acid material has very good rate performance. The following figure shows the cycle performance curve of this material. It can be seen from the figure that at a current density of 2.31 A/g, 500 cycles, the capacity retention rate of the material is 98.1%, which is even better than that of artificial graphite. . The morphology of maleic acid in different electrochemical states is shown in the figure below. From the picture, it can be seen that when the battery voltage drops to 0.8V, an insoluble surface film appears on the surface of maleic acid particles. It may be due to the reaction of maleic acid with solvated Li. The swelling of 0.01V maleic acid particles is caused by the formation of lithium intercalation and SEI film. During the charging process, the battery was charged to 1, 2, and 3V electrode morphology did not change significantly, indicating that the morphology of maleic acid during the cycle is very stable. Yan Wang used XPS to study the reaction mechanism of maleic acid in detail. He concluded that the reaction process of maleic acid is shown in the figure below. The overall reaction process is divided into five steps, and the final reaction results in structure 7 compounds. It will produce irreversible products such as Li2O, so there will be eight electrons involved in the reversible reaction throughout the reaction process, but the actual capacity of maleic acid is 1500mAh/g, so there should be twelve electrons involved in the reversible reaction, so Yan Wang thinks There are also some surface reactions of maleic acid involved in the electrochemical reaction, providing capacity for the material. As a new type of lithium ion battery anode material for organics, maleic acid exhibits extremely excellent electrochemical performance. It not only has high capacity (1500mAh/g reversible capacity), excellent cycle performance (recycle capacity of 500 cycles is 98.1%), but also With amazing rate performance (46.2A/g current density, the specific capacity of maleic acid can reach 570mAh/g), it is very suitable for application on some high specific energy, high power batteries, the low cost of maleic acid, I believe It will revolutionize the lithium-ion battery industry. Towel Rack,Square Robe Hook,Towel Bars,Robe Hook FOSHAN SHAMANDA SANITARY WARE CO., LTD , https://www.shamandahome.com