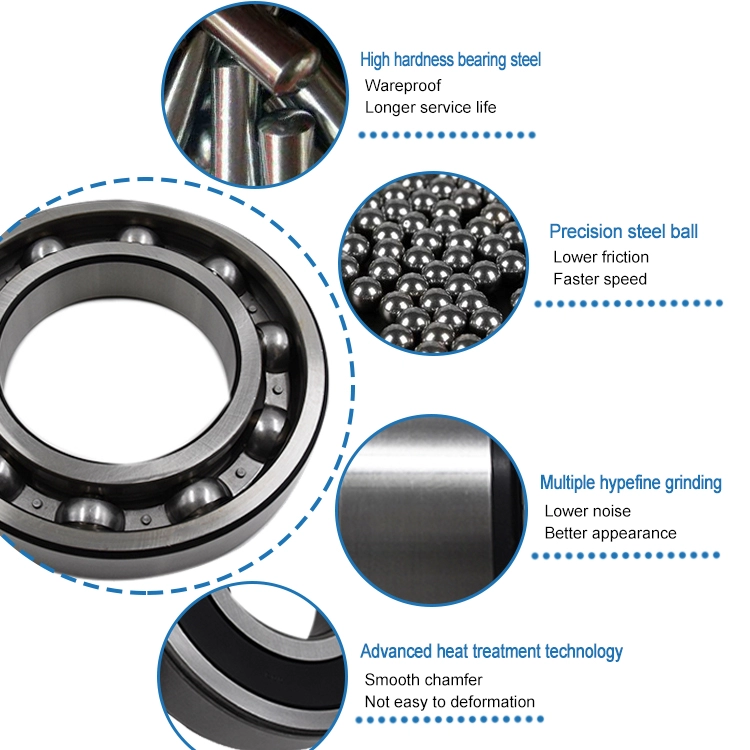
Deep groove ball bearings The out looks of them are nearly the same,the only different is the size,you may need other models please check the following table for reference.are the most widely used bearing type and are particularly versatile.
Deep groove ball bearings are divided into the following series: 6000series, 6100series, 6200series, 6300series, 6400 series, 6700 series, 6800 series,6900 series,16000 series etc.
1.Small bearings|:plastic tube+carton+pallet High Ball Bearing,Miniature Ball Bearing,Deep Groove Ball Bearing,Deep Ball Bearing Shijiazhuang Longshu Mechanical & Electrical Equipment Trading Co., Ltd. , https://www.longsbearings.com
- ammonium sulfate
Ammonium sulphate (21-0-0-24S) is a long-established and most common sulfur-containing nitrogen fertilizer and is still widely used. Ammonium sulfate is mostly a by-product of other industries. It is estimated that 70% of the world's ammonium sulfate is a by-product of the production of synthetic fiber intermediate caprolactam, a small part of which is recovered from coke oven gas, and the rest is produced from sulfuric acid and synthetic ammonia. In 2002, the world's ammonium sulfate production was about 17.5 million tons, equivalent to 4.2 million tons of sulfur, accounting for 30% of the total sulfur use. About 3 million tons of sulfur fertilizer is applied directly, and the rest is applied in the form of various compound fertilizers. The improved ammonium sulphate production process produces large particle ammonium sulphate suitable for bulk blended fertilizer production. This greatly increases the choice of use and facilitates the production of compost. Ammonium sulfate is also widely used in the production of compound fertilizers in Western Europe. Sulfur ammonium is currently being consciously added to increase the sulfur content in the compound fertilizer.
The main advantages of ammonium sulphate are low hygroscopicity and strong chemical stability, which are excellent sources of nitrogen and sulfur. Ammonium sulfate is a physiologically acidic reaction in the soil, which is beneficial for use in soils with high pH and acidic crops. Ammonium sulfate is not suitable for acidic soils that already require lime neutralization. When ammonium sulphate is applied, the supply of nitrogen to the soil also adds more sulfur to the soil than is required for most crops. In addition to its nitrogen to sulfur ratio imbalance, frequent high rates of ammonium sulfate promote acidification of poorly buffered soils.
There are currently granular ammonium sulphate products. This product is easy to transport and suitable for blending with other fertilizers. In the production of fertilizers, if the particle size of the product does not match the other nitrogen, phosphorus, potassium fertilizer raw materials, the phenomenon of separation will occur. This problem can be solved by controlling the uniformity and particle size of the particulate ammonium sulphate product used in bulk blended fertilizers.
Ammonium sulfate can also be used to produce clear liquid fertilizers containing nitrogen and sulfur. The sulfur content in the ammonium sulfate-urea solution ranges from 1% to 9%. In the formulation of NPS liquid fertilizer using ammonium sulfate as a raw material, the sulfur concentration is generally from 1% to 3%.
——Nitrate-ammonium sulfate
The US International Fertilizer Development Center produces two grades of ammonium nitrate-ammonium products with specifications of 30-0-0-5S and 27-0-0-11S. Of the two products, the former is more common and contains 21% (NH4)2SO4 and 79% NH4NO3. Both products are granulated products produced by neutralizing ammonia with nitric acid and sulfuric acid. Nitrogen-ammonium sulfate has several advantages: its hygroscopicity is lower than that of ammonium nitrate and ammonium sulfate, and the ratio of nitrogen to sulfur is more suitable for direct application. It also contains ammonium nitrogen and nitrate nitrogen. It has been successfully applied directly to pasture and cereal crops in the United States, and is very effective.
——Urea-ammonium sulfate
Granular urea-ammonium sulfate can be produced by encapsulating fine powder ammonium sulfate in the granulator and the spray granulation tower, and the specifications are 40-0-0-14S to 31-00-13S. Urea-ammonium sulfate has stronger anti-pulverization performance and less hygroscopicity than urea granules, and its physical properties can be further improved by adding gypsum to urea to form a complex. The product's nitrogen to sulfur ratio ranges from 3:1 to 7:1, which is more flexible for most nitrogen- and sulfur-deficient soil applications. The acidification reaction of ammonium sulphate in the soil reduces the activity of urinary enzymes and reduces the loss of ammonia by inhibiting the hydrolysis of urea to alleviate the increase in pH.
Products with a specification of 34-0-0-11S made by mechanically blending urea and ammonium sulphate have been sold in western Canada since 1967 and are currently in good condition. Since the particle size of the ammonium sulfate product is not ideal for segregation, the blended fertilizer product should be used as soon as possible after preparation.
- ammonium phosphate sulfate
The most common commercial ammonium phosphate ammonium has a specification of 16-9-0-14S (16-20-0-14S) and consists of about 40% monoammonium phosphate and 60% ammonium sulfate. Other specifications for this type of product are 13-16-0-20S (13-39-0-20S), 19-4-0-20S (19-9-0-20S), and 23-9-0-7S ( 23-20-0-7S), the latter also contains some urea. There are several production processes, such as reacting a mixed acid of phosphoric acid and sulfuric acid with ammonia, and adding an ammonium sulfate solution and sulfuric acid to the reaction system of the phosphoric acid production unit. In many countries, 16-9-0-14S (16-20-0-14S) is applied directly to pasture crops and legume crops, and it is also common in cereal and rapeseed fields. This product is also often used as a base material for the production of bulk blended fertilizers. At present, the granular product produced in the United States has a specification of 15-3-0-8S (15-30-0-8S), and the raw materials are ammonium phosphate, ammonium sulfate and gypsum. The product is suitable for the production of blended fertilizer and direct use.
In 1993, China developed a new process for the production of sulfur-based NPK compound fertilizers using phosphate rock, sulfuric acid, ammonia, urea and potassium chloride as raw materials. The new process combines the three processes of producing ammonium sulfate, potassium salts and NPK(S), which greatly simplifies the process and reduces production costs. The product contains N14.5%, P2O5 16%, K2O 14.5%, S11% (ammonium sulfate and potassium sulfate). At present, the production capacity of China's sulfur-based NPK compound fertilizer has increased to 5.68 million tons. In 2002, the production of sulfur-based NPK compound fertilizer reached 4.6 million tons, and sulfur supply was 500,000 tons. It is expected that the production capacity of sulfur-containing compound fertilizer will increase to 7 million tons. Above t, it is equivalent to providing 700,000 tons of sulfur.
- Gypsum (CaSO4•2H2O)
Gypsum (18S) is used as a source of sulfur in areas where there are minerals or by-products, and its content is low, generally 13%-19% sulfur, which limits its commercial use. However, the application of gypsum can provide calcium to the crop or improve the soil. Gypsum is widely used in blended fertilizers and as a source of sulfur and calcium directly on peanuts.
- Calcium
Calcium was once the most important phosphate fertilizer in the world, and because it contains both phosphorus and sulfur, it is still one of the major phosphate fertilizer varieties in China, India, Australia and New Zealand. 50% of the weight of calcium is calcium monocalcium phosphate and gypsum (water gypsum and dihydrate gypsum), phosphorus 6%-9.5% (P2O5 12%-22%) and 10%-14% sulfur, which is an excellent Source of sulfur. In many parts of the world, in the past, a large amount of calcium was added to supplement phosphorus, and sulfur was added unconsciously, delaying the occurrence of sulfur deficiency. Calcium also contains 18% to 21% calcium, which is important in the absence of calcium in the soil.
In the 1960s, the structure of the world phosphate fertilizer industry changed. High-concentration phosphate fertilizers, such as diammonium phosphate, replaced calcium. At the same time, it was more economical to produce ammonium phosphate in areas rich in phosphate resources than in producing calcium in agricultural areas. The production of calcium is declining. The vast majority of new phosphate fertilizer production facilities in the world are being planned for ammonium phosphate rather than calcium. But this may also be a factor that is conducive to the consumption of raw material sulfur and exacerbates soil sulfur deficiency. However, due to low production energy consumption, the availability of sulfuric acid from other industrial by-products, and the increased demand for sulfur and trace element fertilizers in agricultural production, the amount of calcium applied will continue to increase in the future. In 2001, 4.1 million tons of sulfur were applied to the soil along with calcium, accounting for 43% of the total sulfur application. China's current annual production of calcium is 3.7 million tons of P2O5. Typical calcium products contain 14% P2O5 and 12% sulfur, which is equivalent to 3.1 million tons of sulfur applied to the soil. China's future annual output of calcium is expected to remain at 3.7 million tons of P2O5, which is still the main source of sulfur in China's soil, accounting for 90% of the total amount of sulfur applied to soil by Chinese fertilizer forms.
- Phosphogypsum
Phosphogypsum is a by-product of the production of wet-process phosphoric acid, producing about 1 t of phosphoric acid by-product of 1 t of phosphoric acid. Since phosphogypsum contains all the sulfur from sulfuric acid, it is an economical source of sulfur available to the local agricultural sector. It is estimated that in 2001, the world's total production of phosphogypsum was 140 million tons, including 25 million tons of sulfur (15%-17% sulfur). In 1982, developing countries in the tropical regions of the Far East, Africa, and Latin America produced a total of 18.6 million tons of phosphogypsum and more than 3 million tons of sulfur. China's annual high-concentration phosphate fertilizer by-product of phosphogypsum is about 100-1.2 million tons. Especially in the case of severe sulfur deficiency in the soils of these countries, they cannot waste this cheap sulfur resource.
Phosphogypsum also contains small amounts of phosphorus and heavy metal impurities from phosphate rock. These characteristics of phosphogypsum are not problematic at all as a use of sulphur fertilizer, and can reduce environmental pollution caused by the accumulation of a large amount of phosphogypsum. The agronomic effects of the use of phosphogypsum as a sulphur fertilizer and the technical issues related to drying, transportation, storage, treatment and conversion of phosphogypsum to AS (ammonium sulphate) or sulphuric acid are required.
- Potassium sulphate (K2SO4)
Potassium sulphate is the main sulphur-containing potassium fertilizer, white granules containing 42%-44% potassium (containing K2O 50%-53%) and 17% sulfur. At present, the market sales volume of potassium sulfate is 3 million tons per year, which is equivalent to about 500,000 tons of sulfur. The production process of potassium sulfate varies depending on the raw materials, and most of the potassium sulfate is directly obtained from potassium salts or brines. Approximately 25% of the potassium sulfate is produced by a process in which potassium chloride is reacted with sulfuric acid. Although potassium sulfate is more expensive than potassium chloride in the market, it is still widely used as a special fertilizer on chloride-sensitive crops such as potatoes and tobacco leaves. Another advantage is that it also supplies sulfur to crops.
- potassium sulphate
Potassium magnesium sulfate is a double salt containing K18% (K2O 22%), Mg 11%, S 22%. The advantage is that it provides both magnesium and sulfur and is widely used in the production of compound fertilizers, which are used to supplement the two elements to the soil when the soil is simultaneously deficient in magnesium and sulfur. It is more desirable to use a chemical fertilizer with a low chloride content. The crops that often use such fertilizers are potatoes, tobacco leaves, peach trees, bean crops, and grasslands.
- Magnesium sulfate and sulfate trace element fertilizer
Magnesium sulfate contains 13% sulfur and 9.8% magnesium. It is used in a limited amount. It is mainly used as a clear source of liquid fertilizer and foliar fertilizer. It also provides a large amount of sulfur while applying magnesium.
Source: Agricultural Network
Deep Groove Ball Bearings
They have low friction and are optimized for low noise and low vibration which enables high rotational speeds.
They accommodate radial and axial loads in both directions, are easy to mount, and require less maintenance than other bearing types.
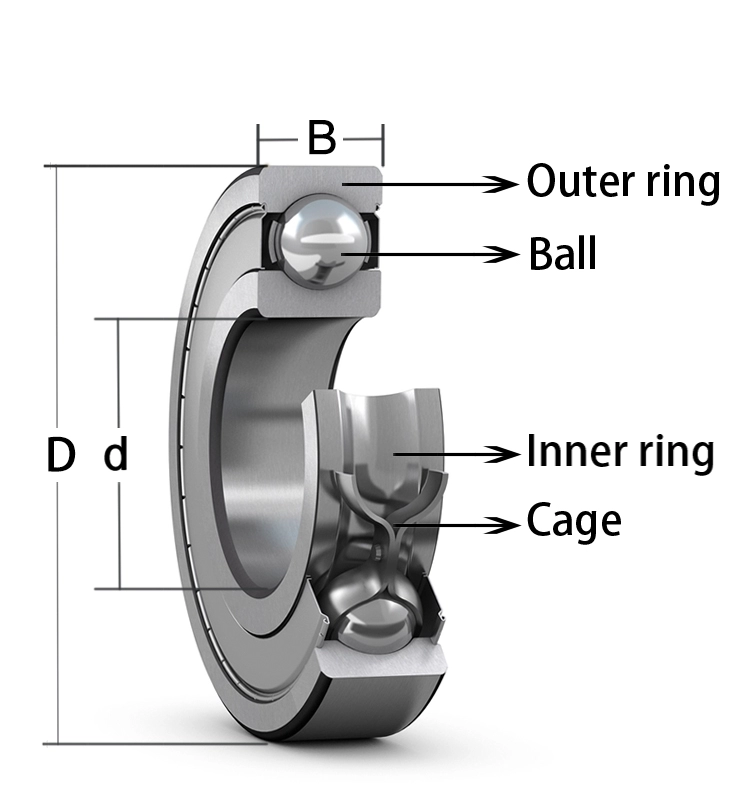
Model
d(mm)
D(mm)
B(mm)
6000ZZ
10
26
8
6001ZZ
12
28
8
6002ZZ
15
32
9
6003ZZ
17
35
10
6004ZZ
20
42
12
6005ZZ
25
47
12
6006ZZ
30
55
13
6007ZZ
35
62
14
6008ZZ
40
68
15


We manage in all kinds of ball bearings and roller bearings,such as
1.Deep Groove Ball Bearing
2.Self-aligning Ball Bearing
3.Cylindrical Roller Bearing
4.Spherical Contact Bearing
5.Angular Contact Bearing
6.Tapered Roller Bearing
7.Thrust Ball Bearing
8.Insert Bearing
Package
2.Medium bearings:PE bag+box+carton+pallet
3.Large bearings:PE film & paper winding+carton+pallet
4.According to the customer's requirements
Sulphate fertilizer variety introduction
The sulphate-containing fertilizer has many varieties and is used in large amounts. It is the fertilizer that supplies the most sulfur to the soil. Its important advantage is that sulfate as a component of the multi-fertilizer provides a form of direct absorption to the crop, SO42-. The most widely used and most common fertilizer varieties are ammonium sulfate, calcium, potassium sulfate, potassium sulphate and gypsum.
ã€Comment】 ã€Print this article】 ã€Close this page】 ã€Large, medium and small】